Neonatal Hearing Screening: Denoising & Feature Extraction of EEG Signal Using Wavelet Transform
Problem Statement:
Concept:
Wavelet transform is a powerful tool for extracting auditory event-related potentials (ERPs) from EEG signals due to its ability to analyse non-stationary signals with high time-frequency resolution. Here’s how it is typically applied:
1. Signal Acquisition
2. Wavelet Decomposition
3. Thresholding and Reconstruction
4. Analysis and Interpretation
Technology/Science involved:
Auditory Brainstem Response (ABR), Otoacoustic Emissions (OAEs), Wavelet Transform, Wavelet Denoising
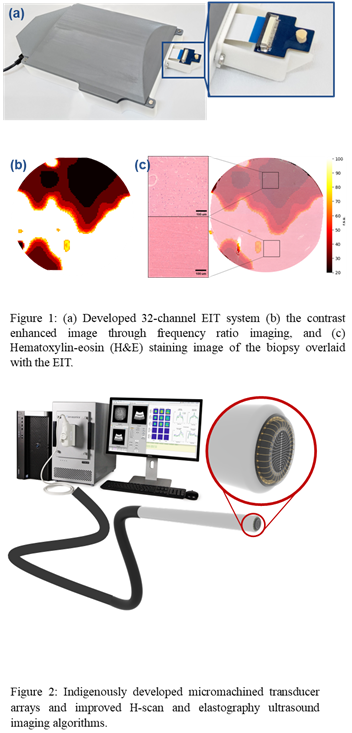
Electro-acoustic characterization of cranial tissues for tumour delineation
Problem Statement:
Concept:
The project involves designing, simulating and fabricating arrays of pMUTs (Piezoelectric Micromachined Ultrasound Transducers) arrays for the desired frequency range. These pMUTs will be packaged on a single chip along with EIT (Electrical impedance tomography) electrodes, and the final device will be integrated into the tip of a probe. This multimodal intraoperative probe would exploit the acoustic and electrical modalities to image the tissue and help in margin delineation during the surgery, thus enabling an efficient resection of the tumour.
Technology/Science involved:
MEMS, Microfabrication, Neurosurgical tools, Material characterisation
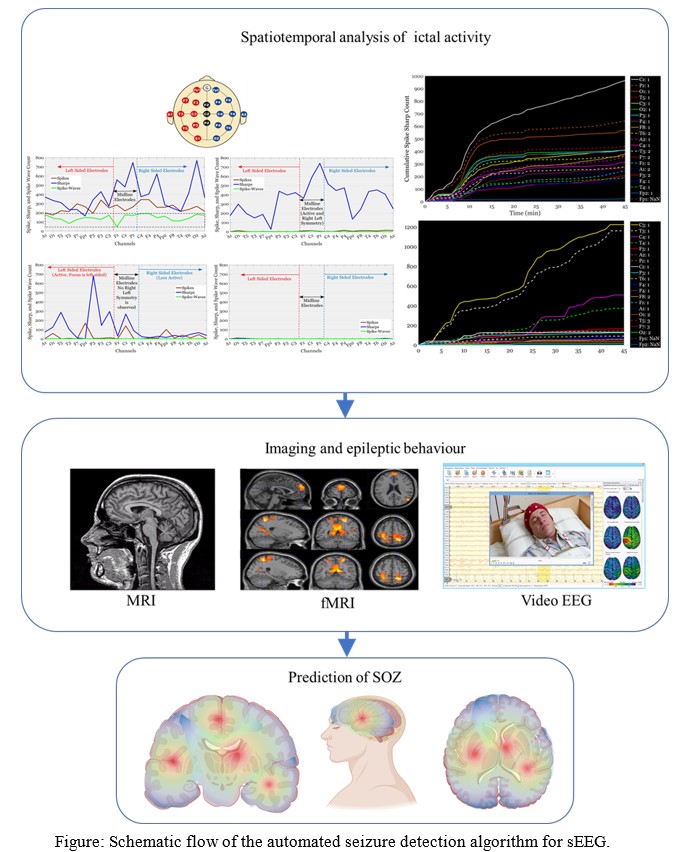
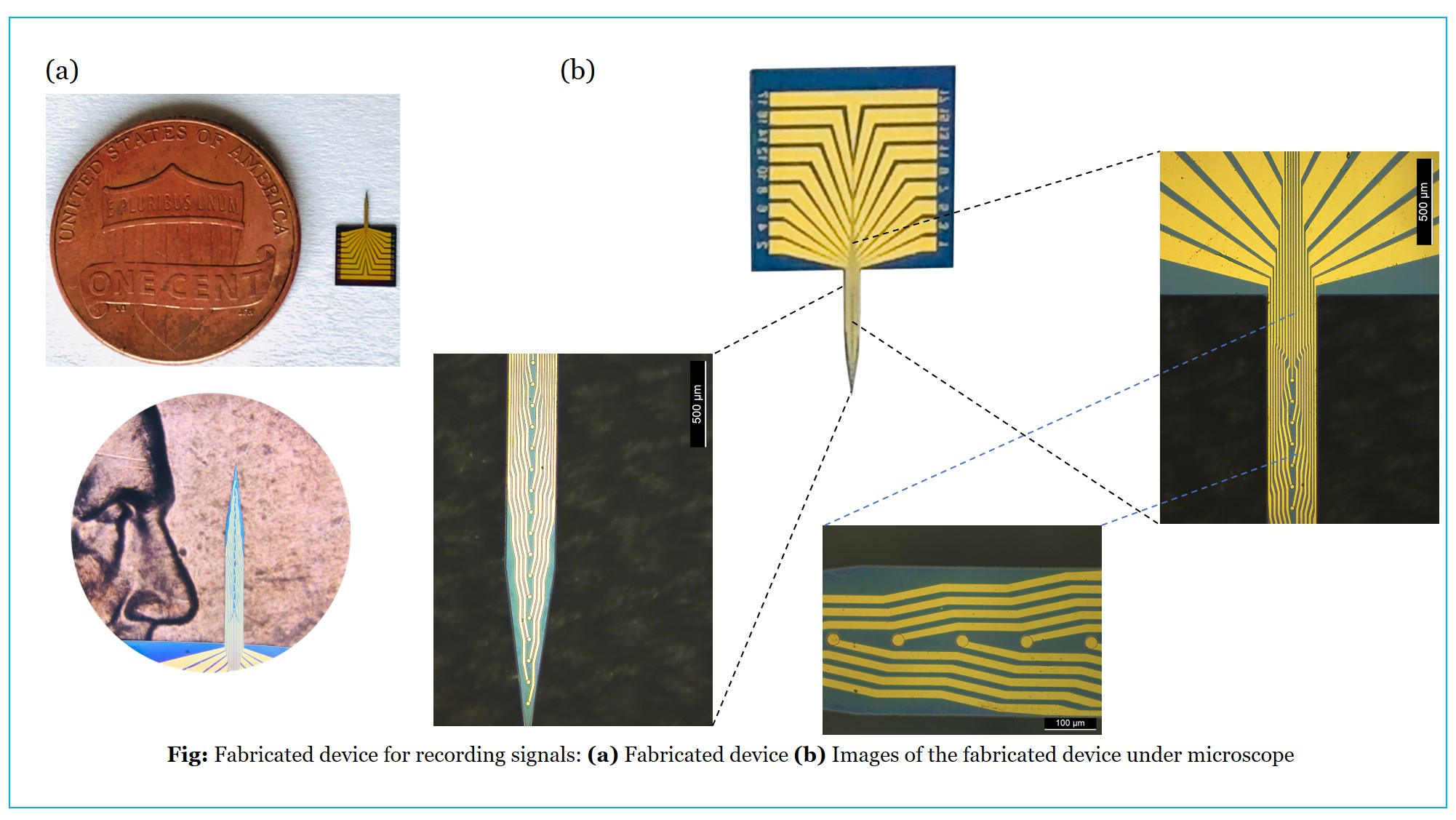
Towards Precise localization of brain activity with sEEG electrodes
Problem Statement:
Stereo Electroencephalography (sEEG) is a specialized neurosurgical method that captures the brain’s electrical activity with exceptional spatial precision. It requires the insertion of multiple-depth electrodes, also known as stereo electrodes, into specific areas of interest within the brain. This procedure is typically performed in cases of intractable or refractory epilepsy, where the individual is entirely unresponsive to Anti-Epileptic Drugs (AED). In such cases, a surgeon must surgically remove the specific brain region causing the electrical disruptions. This process necessitates a neurophysiologist’s manual examination of the electrophysiological signals, including a manual review of the neural traces, to make deductions about the seizure’s onset and spread. Once the seizure’s location is identified, the surgeon must meticulously plan the removal of the affected part from the brain, ensuring no harm to the surrounding healthy tissue. The electrodes’ resolution and size are critical in this process; the smaller the electrode, the superior the spatial resolution. Therefore, the primary goals of the research are:
- The fabrication of miniature depth electrodes for rodent models
- The development of an automated algorithm for accurately identifying the seizure onset zone and mapping the onset and progression.
Concept:
Accurate pinpointing of the onset and spread of seizures is crucial for mapping the brain area involved in abnormal activity. The electrophysiological variations captured in the sEEG signals must be processed using various filters and thresholds to detect the brain’s electrical disturbances. An automated signal-processing algorithm can address the issue of manually identifying the Seizure Onset Zone (SOZ), offering improved accuracy, reduced time, and less effort for the neurophysiologist and surgeon. The algorithm includes setting thresholds for signal parameters and co-registering the signal onto a 3D model of the patient’s brain. Micro-electro-mechanical system (MEMS)-based methods are ideal for reducing size and increasing the number of contacts in the electrode. This is achievable with MEMS-based fabrication techniques such as coating and thin-film deposition. The electrodes are constructed with gold depositions and passivation layers, which are accurately patterned using photo-lithography and other optics-based methods. This reduces the electrode’s size to one-tenth of commercially available electrodes (approximately 70 micrometers).
Technology/Science involved:
Neurophysiology, Neural interface, Signal processing, MEMS-devices
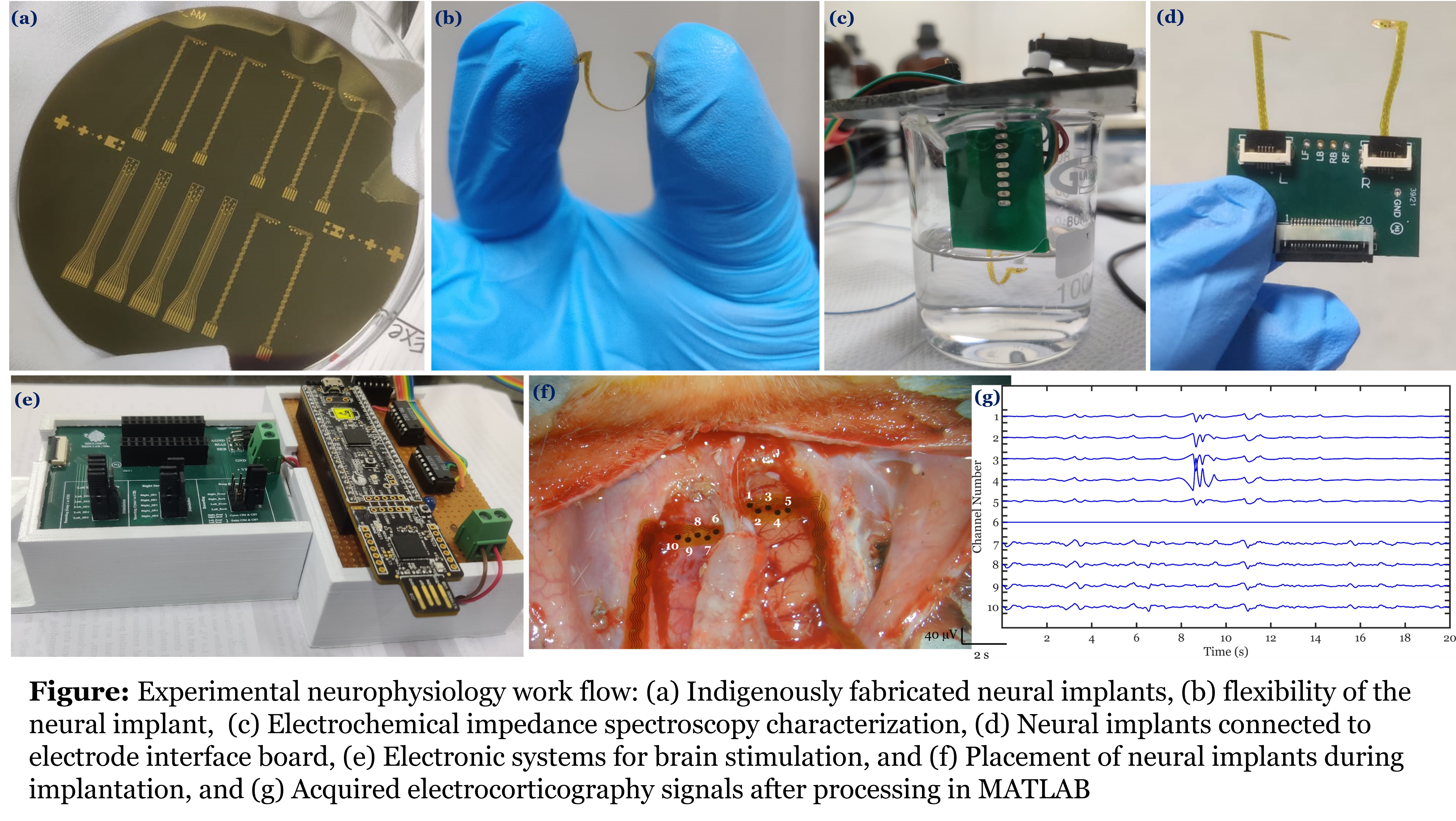
Multifunctional transparent electrode array for in-vivo delineation of tumour/stroke from normal
Problem Statement:
Studies have shown that the tumour and stroke tissues have different electrical properties with respect to the adjacent healthy tissue. The variations in these properties can be contributed to higher water content in tumour tissues, elevated or decreased ion concentrations, and even dielectric behaviour of cell membranes. Neural tissues being excitable can be electrically stimulated and functional responses can be produced to identify the eloquent cortexes. Alternatively, action potentials can be recorded to know the functional regions of brain. In this work, we explore different electrical behaviors of brain tissue to delineate tumour/stroke margin.
Concept:
Flexible, transparent electrode array is designed and fabricated to use 3 modalities for in-vivo tumour/stroke margin delineation. Electronic modules to carry out the study using three modalities namely (a) Direct Electric Stimulation (DES) (b) Electrical Impedance Spectroscopy (EIS) (c) Electrocorticography (ECoG) are also developed inhouse.
Technology/Science involved:
MEMS-based devices, Neurophysiology, Neural interfaces, and Electronics
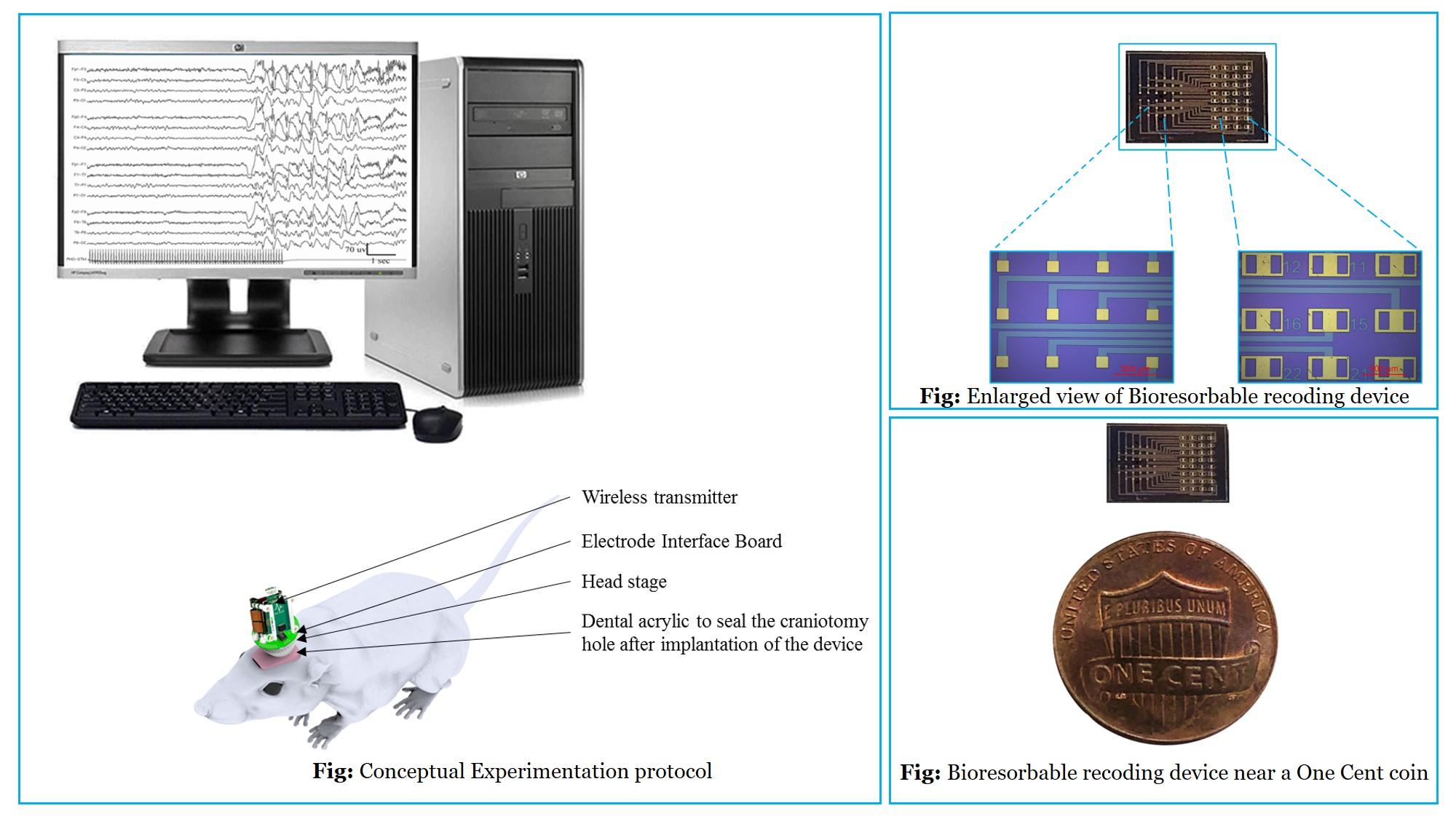
Minimally invasive Bioresorbable Devices for Anti-epileptic Drug Screening by Recording ECoG Signals
Problem Statement:
Epilepsy is one of the most common neurological disorders characterized by abnormal, excessive, and hypersynchronous activity of brain. However, there is no definitive epilepsy-specific drug for treatment. Moreover, intractable epilepsy (~30% of the patients) cannot be treated with anti-epileptic drugs (AEDs). It requires mapping of brain surface to detect the source followed by surgery. Continuous recording of the brain signals at cortical surface requires implantable devices. Conventional implantable devices for monitoring signals require surgical retrieval procedure that may lead to trauma and further complications.
Concept:
Bioresorbable materials get dissolved in body fluids based on pH and temperature. Thus, such materials can be used to engineer a novel class of devices that get resorbed within the body eliminating the shortcomings of conventional implantable devices. We are working on such devices that can be implanted on the brain to record high density electrocorticography (ECoG) signals (normal with ictal and inter-ictal signals) for detecting the epileptic focus and for anti-epileptic drug screening. These MEMS-based bioresorbable sensors can be fabricated using existing microfabrication technologies.
Technology/Science involved:
MEMS, Neurophysiology, Acute and chronic models of epilepsy, Anti-epileptic drug screening
Micro-Electrode Cannula Arrays to Design Neuroprotective Therapies for Acute Stroke and Epilepsy
Problem Statement:
The absence of physiologically realistic models of brain diseases have resulted in failure to translate promising animal results into therapy. Appropriate measurement of neuronal function is critical for discovering therapeutic interventions. Realistic physiological models and stringent neurophysiological metrics play an important role in the development of therapies for brain diseases.
Concept:
The therapies can be validated using realistic acute neurophysiological models where pathology and metrics faithfully represent the electrophysiological disturbances in humans. Electrophysiological disturbances in the brain can be studied by recording electrocorticography and stereo-electrocorticography signals. The electrode arrays will be implanted in vivo (after craniotomy) to measure extracellular neo-cortical and column level Local Field Potential (LFP) responses. The acute stroke and epilepsy models in rats will be studied by recording the neo-cortical circuit activity as Local Field Potentials from deep brain regions using fabricated intra-cortical micro-electrode arrays.
Technology/Science involved:
MEMS, Neurophysiology, Neuroprotective therapies, Acute models to study neurological disorders
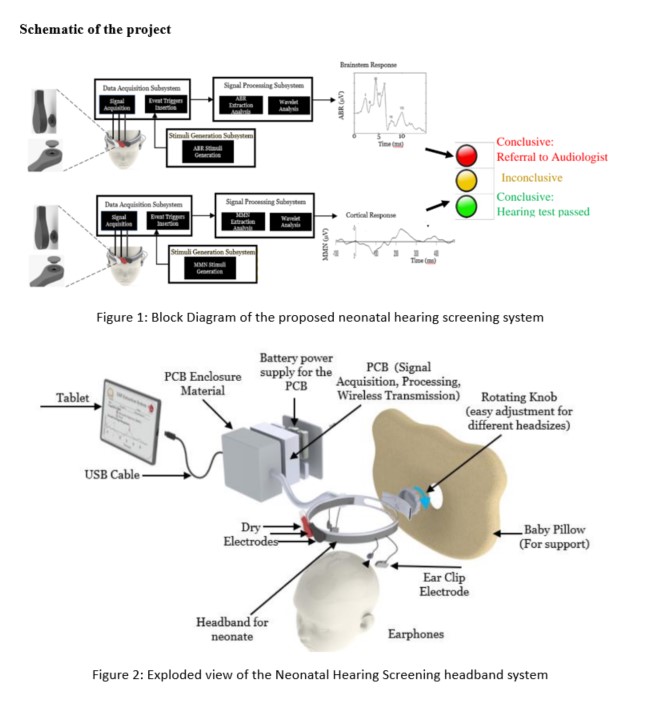
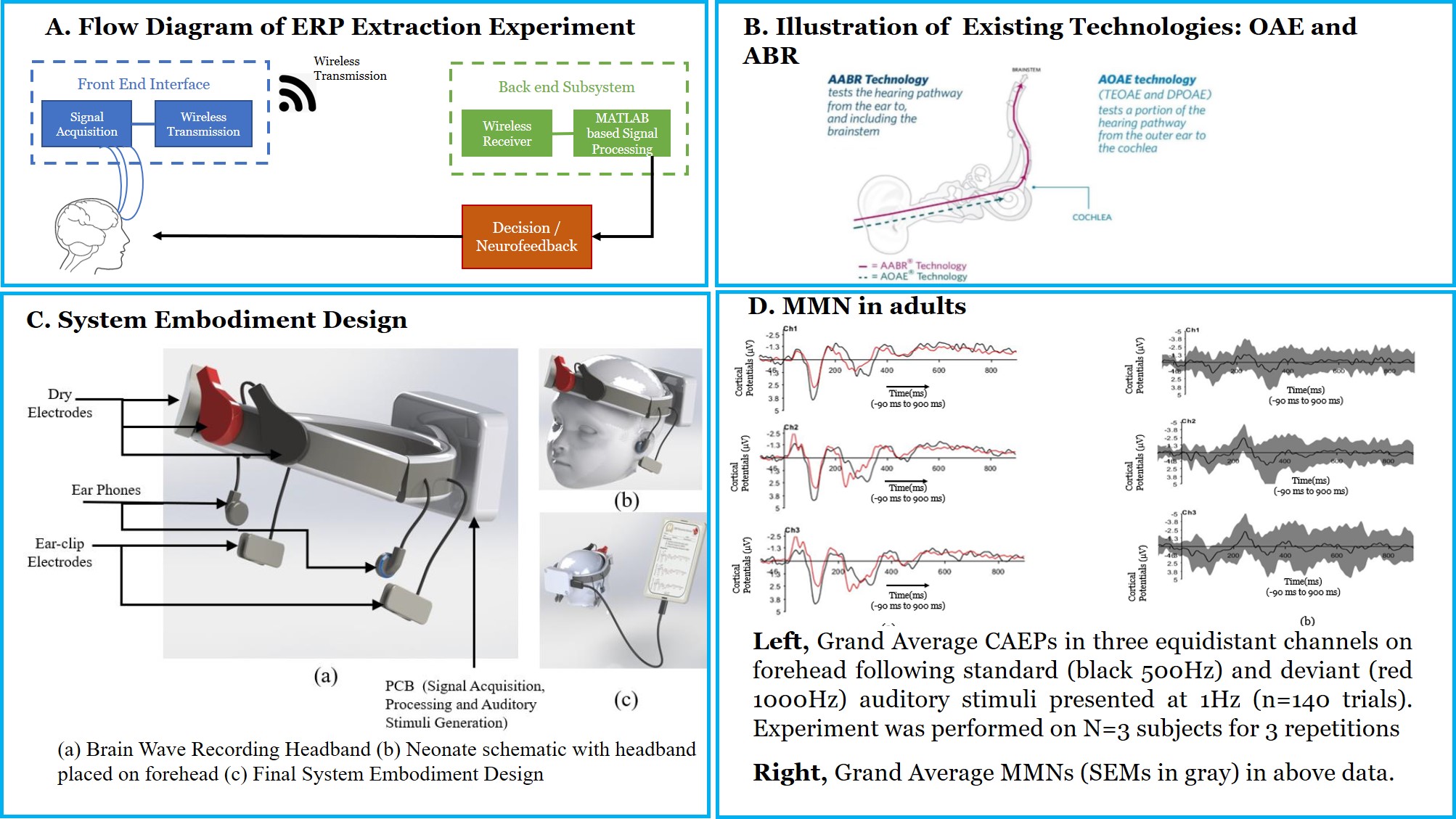
Rapid neonatal hearing screening (NHS) devices using the Cortical Auditory Evoked Potential (CAEP) and the Mismatch Negativity (MMN) Event-Related Potential (ERP)
Problem Statement:
Neonatal hearing loss is the most common sensory deficit present at the time of birth. Congenital deafness has severe repercussions including difficulties in speech perception, delayed language acquisition, lack of auditory attention, verbal memory and hence literacy skills. Nevertheless, if hearing loss is detected early, infants can receive early intervention and many negative consequences can be eliminated or reduced. Therefore, timely detection and intervention is a crucial area of hearing screening research. Auditory Brainstem Response (ABR) and Otoacoustic Emissions (OAE) are the current approaches to detect deafness in neonates. Both methodologies test a specific early part of the auditory pathway, but not its entirety until the perception of sound in the brain. Moreover, the paucity of the clinicians or audiometric professionals, expensive equipment, lack of specialized hospitals with audiometry tools and patient follow up are the challenges for the success of current Neonatal Hearing screening programs, especially in a resource-constrained country like India.
Concept:
ABR and OAE checks a specific portion of the auditory pathway. Moreover, ABR and OAE do not scan the auditory pathway beyond brainstem region. Perception of sound involves the entire sensory pathway. Studies have confirmed that Cortical Auditory Evoked Potentials are a comprehensive signature for hearing deficits. Lack of a complete scan of the auditory pathway is the key gap in current newborn screening methodologies. We aim to address this gap by measure cortical auditory evoked potential, Mismatch Negativity (MMN). This work aims to overcome the current limitations by checking the complete auditory pathway in a robust, reliable and cost-effective way. Additionally, project will potentially prevent congenital deafness, paving the way for advancement towards a new class of non-invasive Neonatal Hearing Screening technologies in India.
Technology/Science involved:
Computational Neuroscience, Electronics System Design, Biomedical Signal Processing, Neurophysiology
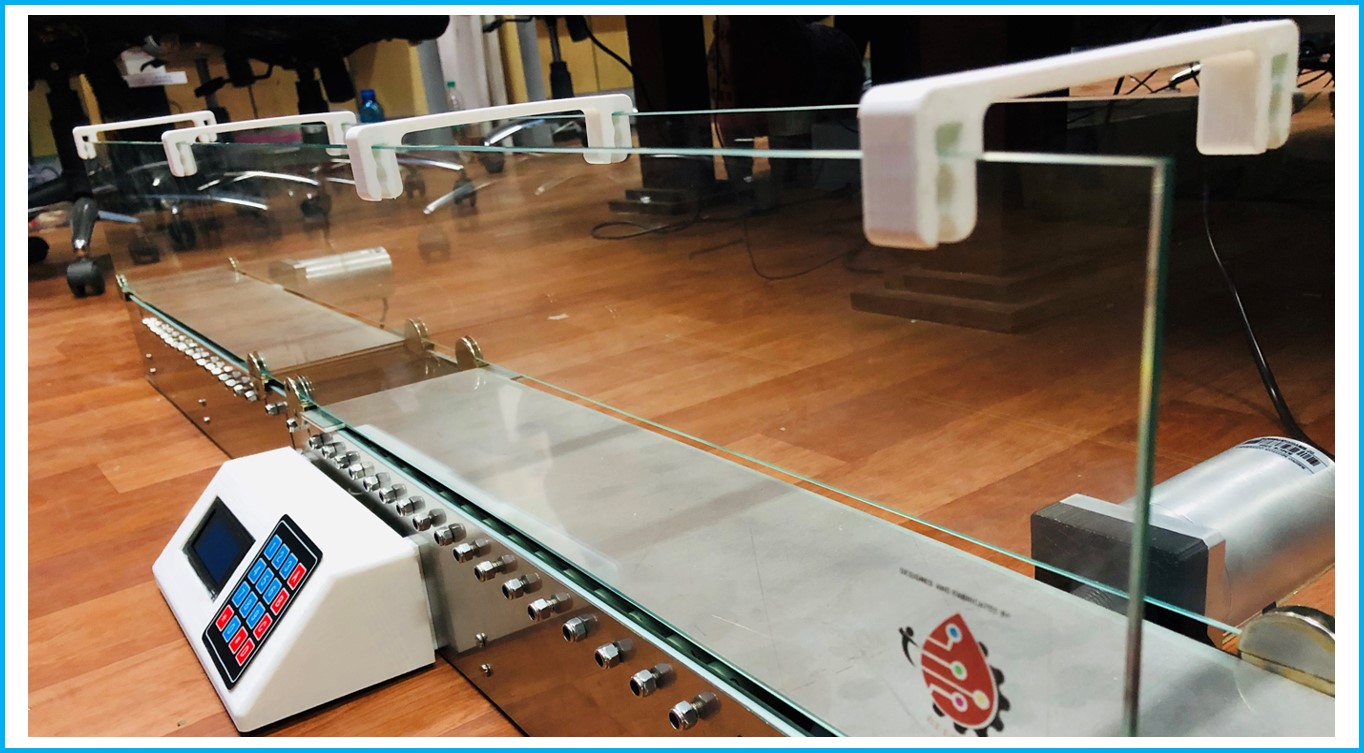
Rat Treadmill for Linear Track Navigation
Problem Statement:
Understanding the Neural responses in rats when subjected to motivated training.
Concept:
Rat treadmills are used for forced exercise training and accurate testing of fatigue in rodents. Developed to understand the rat behavior on the treadmill and motivated to run through the track to collect the reward placed at the other end of the track. The reward will be activated once the rat reaches the reward point and stays there for a stipulated duration. Neural recordings will be taken from the rat parallelly to understand the physiological effects during experiment.
Applications:
Typical Applications include studying:
- Behavioral
- Physiological
- Biochemical
- Molecular responses to both acute exercise stress and chronic exercise training.
Design and Development of Micro-engineered Electrodes for Parkinson's disease in Rat models
Problem Statement:
To compare the outcomes of Deep Brain Stimulation (DBS) and surface brain stimulation in Parkinsonian rat models.
Concept:
Deep Brain Stimulation of the Subthalamic nucleus (STN) in the human brain is a well-established clinical therapy for advanced Parkinson’s disease (PD) patients. However, it has several side effects, such as psychological disorders, headaches, etc. Some symptoms related to freezing of gait are not improved in STN-DBS. An alternate deep brain region needs to be explored for this purpose. Also, DBS is an invasive surgery, in which the DBS electrodes are implanted in the deep brain regions. In this research, several surface brain regions will be stimulated, and their outcome will be compared with the DBS to see if the invasiveness of the surgery can be reduced.
Technology/Science involved:
MEMS-based fabrication processes, Electrochemistry, Electronics System Design, In vivo experimentation, Neurophysiology, Neural Signal Processing, and Behavioral studies